"Understanding the Amide Group: Structure, Properties, and Applications"Amides are an essential class of organic compounds with wide-ranging applications in biochemistry, medicine, and industrial chemistry. The amide group is a functional group characterized by its unique structure and chemical behavior. This topic explores the amide group, its properties, types, and significance in various fields.
What Is an Amide Group?
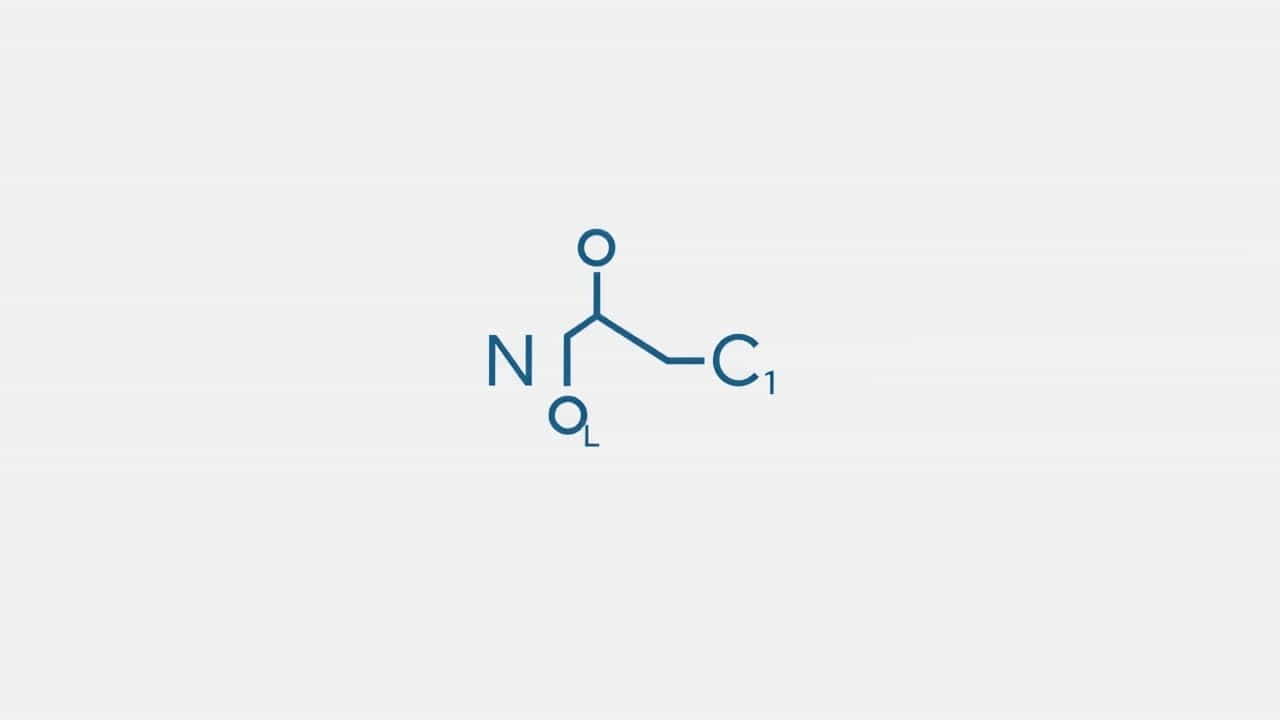
An amide group is a functional group consisting of a carbonyl group (C=O) bonded to a nitrogen atom (-NH₂, -NHR, or -NR₂). It can be represented as R-C(=O)-NR₂, where R represents an alkyl or aryl group and R₂ represents hydrogen or hydrocarbon chains.
Structure of the Amide Group
-
Carbonyl Group: The carbon atom is double-bonded to an oxygen atom, making it electrophilic.
-
Nitrogen Atom: The nitrogen in the amide group is connected to the carbonyl carbon, and it can carry hydrogen atoms or additional substituents.
-
Planarity: Due to resonance, the amide bond has partial double-bond character, resulting in a planar and rigid structure.
Types of Amides
Amides are classified based on the substituents attached to the nitrogen atom:
1. Primary Amides
Primary amides have one hydrogen atom bonded to the nitrogen atom.
Example: Formamide (HCONH₂).
2. Secondary Amides
Secondary amides have one alkyl or aryl group and one hydrogen atom attached to the nitrogen atom.
Example: Acetamide (CH₃CONH).
3. Tertiary Amides
Tertiary amides have two alkyl or aryl groups attached to the nitrogen atom.
Example: Dimethylacetamide (CH₃CON(CH₃)₂).
Properties of Amides
Amides exhibit a range of physical and chemical properties due to their structure.
Physical Properties
-
Polarity: Amides are polar compounds due to the carbonyl and nitrogen groups.
-
Hydrogen Bonding: Primary and secondary amides can form hydrogen bonds, leading to higher boiling points compared to other functional groups.
-
Solubility: Lower amides are soluble in water because of their ability to form hydrogen bonds with water molecules.
Chemical Properties
-
Stability: Amides are relatively stable due to resonance stabilization between the carbonyl group and the nitrogen atom.
-
Acidity and Basicity: Amides are neutral compounds, although they exhibit weak acidic or basic behavior in specific reactions.
-
Hydrolysis: Amides can undergo hydrolysis to form carboxylic acids and amines in the presence of acids or bases.
Formation of Amides
Amides are typically formed through reactions involving carboxylic acids or their derivatives.
1. From Carboxylic Acids and Amines
Carboxylic acids react with amines to produce amides in the presence of dehydrating agents.
Example:
RCOOH + NH₃ → RCONH₂ + H₂O
2. From Acid Chlorides
Acid chlorides react with amines to form amides under mild conditions.
Example:
RCOCl + R’NH₂ → RCONHR’ + HCl
3. From Esters
Esters can be converted to amides through aminolysis, where the ester reacts with an amine.
Example:
RCOOR’ + NH₃ → RCONH₂ + R’OH
Reactions of Amides
Amides are versatile compounds that participate in several chemical reactions.
1. Hydrolysis
Amides can be hydrolyzed in acidic or basic conditions to form carboxylic acids and amines.
Example:
RCONH₂ + H₂O + H⁺ → RCOOH + NH₄⁺
2. Reduction
Amides can be reduced to amines using reducing agents like lithium aluminum hydride (LiAlH₄).
Example:
RCONH₂ + 4[H] → RCH₂NH₂
3. Dehydration
Amides can undergo dehydration to form nitriles when treated with dehydrating agents.
Example:
RCONH₂ → RCN + H₂O
Significance of Amide Groups in Biological Molecules
The amide group plays a crucial role in biochemistry, particularly in the structure and function of proteins.
1. Peptide Bonds
Amide bonds, also known as peptide bonds, link amino acids together to form proteins. The rigidity of the amide bond contributes to the structural stability of proteins.
2. Enzyme Function
Enzymes, which are proteins, rely on amide bonds for their catalytic activity. The specific arrangement of amide bonds determines the active site’s shape and function.
Applications of Amides
Amides have diverse applications in industrial processes, pharmaceuticals, and materials science.
1. Pharmaceuticals
Amides are found in many drugs, including paracetamol and penicillin. Their stability and bioavailability make them essential in drug design.
2. Polymers
Polyamides, such as nylon, are widely used in textiles, automotive parts, and industrial components due to their strength and durability.
3. Solvents
Certain tertiary amides, like dimethylformamide (DMF) and dimethylacetamide (DMAc), are used as solvents in chemical reactions.
4. Agrochemicals
Amides are used in the synthesis of herbicides, pesticides, and fertilizers.
Distinguishing Amides from Other Functional Groups
Amides can be differentiated from other functional groups based on their unique properties:
-
Amides do not react with sodium bicarbonate, unlike carboxylic acids.
-
Amides are less reactive than acid chlorides, esters, and anhydrides due to resonance stabilization.
Safety Precautions When Handling Amides
-
Handle amides in well-ventilated areas, especially volatile ones, to avoid inhalation.
-
Use gloves and goggles to prevent skin and eye contact.
-
Store amides in cool, dry places away from strong acids and bases.
The amide group is a fascinating and versatile functional group with significant importance in organic chemistry, biology, and industry. Its unique structure and reactivity make it a cornerstone in the synthesis of polymers, pharmaceuticals, and various organic compounds. Understanding the properties and behavior of amides not only deepens our knowledge of chemistry but also highlights their critical role in shaping the modern world.