What Is Fluorine’s Electron Configuration? A Complete Guide for BeginnersFluorine is one of the most important elements in the periodic table. It is well known for being the most reactive of all the halogens and one of the most electronegative elements in existence. Understanding fluorine’s electron configuration is essential for students, chemistry enthusiasts, and anyone interested in how atoms behave. In this topic, we will explore what fluorine’s electron configuration is, how it is arranged, why it matters, and how it influences the properties and uses of fluorine in the real world.
Basic Information About Fluorine
Before we dive into the electron configuration of fluorine, let’s look at its basic characteristics:
-
Element Name: Fluorine
-
Chemical Symbol: F
-
Atomic Number: 9
-
Atomic Mass: 18.998
-
Group: 17 (Halogens)
-
Period: 2
-
State at Room Temperature: Gas
-
Color: Pale yellow
-
Odor: Pungent and irritating
What Is the Electron Configuration of Fluorine?
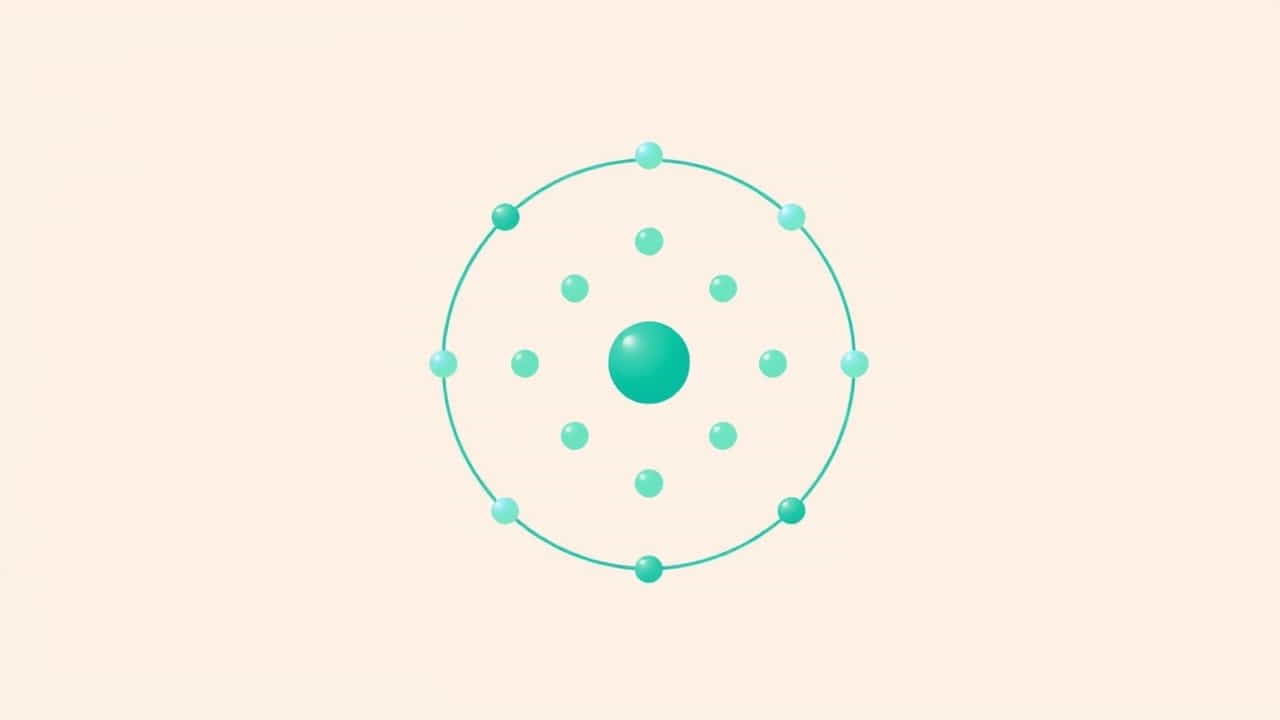
The electron configuration of fluorine describes how its electrons are distributed among different atomic orbitals. Fluorine has an atomic number of 9, which means it has 9 protons and, in a neutral atom, 9 electrons.
The electron configuration of fluorine is:
1s² 2s² 2p⁵
Let’s break that down:
-
The first shell (energy level 1) has 2 electrons in the 1s orbital.
-
The second shell has 2 electrons in the 2s orbital and 5 electrons in the 2p orbitals.
-
That makes a total of 9 electrons, perfectly matching the atomic number of fluorine.
Visualizing Fluorine’s Electron Configuration
If you imagine the electron shells:
-
First shell: 2 electrons (1s²)
-
Second shell: 7 electrons (2s² 2p⁵)
The outermost shell of fluorine has 7 electrons, which is one electron short of having a full octet. This is why fluorine is so reactive it is highly motivated to gain one more electron to fill its outer shell.
Orbital Diagram of Fluorine
In an orbital diagram, we show electrons as arrows filling boxes (orbitals):
-
1s: ↑↓
-
2s: ↑↓
-
2p: ↑↓ ↑ ↑ (leaving one space for a single electron, showing the tendency to fill that orbital)
This diagram confirms that fluorine has five electrons in the 2p orbital and is one electron away from having a completely filled second shell.
Why Is Fluorine So Reactive?
The reactivity of fluorine is directly linked to its electron configuration. With 7 valence electrons (electrons in its outermost shell), fluorine is desperate to gain one more electron to achieve a stable, full octet. This strong tendency to gain an electron makes fluorine extremely reactive and the most electronegative element on the periodic table.
Fluoride Ion Formation
When fluorine gains one electron, it forms a fluoride ion (F⁻) with a full outer shell of 8 electrons. The electron configuration of fluoride ion becomes:
1s² 2s² 2p⁶
This matches the electron configuration of neon, a noble gas, showing that the fluoride ion is very stable.
Electron Configuration in Noble Gas Notation
In noble gas notation, fluorine’s electron configuration is written using the configuration of the nearest noble gas, helium (He), plus the remainder:
[He] 2s² 2p⁵
This simplifies the notation while still showing the distribution of electrons in the outer shell.
Relationship Between Electron Configuration and Chemical Properties
The electron configuration of fluorine explains many of its properties:
1. High Reactivity
Fluorine will aggressively react with almost any other element to gain that final electron.
2. Strong Oxidizing Agent
Fluorine’s desire to gain an electron makes it an excellent oxidizing agent, able to strip electrons from other atoms.
3. High Electronegativity
Fluorine has the highest electronegativity of any element, with a value of 3.98 on the Pauling scale, reflecting its strong pull on electrons in a bond.
4. Formation of Ionic and Covalent Bonds
Fluorine can form ionic bonds (especially with metals, where it takes an electron) or covalent bonds (sharing electrons with nonmetals).
Fluorine in the Periodic Table
Fluorine belongs to the halogen group (Group 17), which consists of elements that all have 7 valence electrons and are highly reactive. Other members include chlorine, bromine, iodine, and astatine. Fluorine is the lightest and most reactive among them, and its electron configuration explains this.
Where Is Fluorine Found?
Fluorine does not exist in its pure elemental form in nature because it is too reactive. Instead, it is found in compounds, most commonly as fluorides in minerals such as fluorite (calcium fluoride). It is also found in trace amounts in water, soil, and even in the human body, where it helps strengthen teeth and bones.
Industrial and Everyday Uses of Fluorine
1. Fluoridation of Water
Small amounts of fluoride are added to drinking water to help prevent tooth decay.
2. Toothpaste
Most toothpastes contain fluoride compounds, which help strengthen tooth enamel and reduce cavities.
3. Teflon Production
Fluorine is used to make polytetrafluoroethylene (PTFE), commonly known as Teflon, a non-stick coating used in cookware.
4. Refrigerants
Fluorine-based compounds, such as hydrofluorocarbons (HFCs), are used in air conditioners and refrigeration systems.
5. Nuclear Industry
Fluorine is used in uranium hexafluoride (UF₆), an important compound in the nuclear fuel cycle.
Safety Considerations
Fluorine gas is extremely dangerous. It is highly toxic, corrosive, and can cause severe burns on contact. Handling fluorine in its pure form is done only by trained professionals under strict safety measures. However, fluoride in small, controlled amounts, such as in water and toothpaste, is safe and beneficial.
Interesting Facts About Fluorine
-
Fluorine was discovered in 1886 by the French chemist Henri Moissan, who won the Nobel Prize in Chemistry for his work.
-
The name fluorine comes from the mineral fluorite, where it was first identified.
-
Fluorine is the most reactive of all elements and can even react with noble gases under certain conditions.
-
Despite its reactivity, fluorine gas has a pale yellow color and is lighter than air.
-
Fluorine is used in the manufacturing of pharmaceuticals, plastics, and even rocket fuels.
How to Write Electron Configuration for Other Elements
Learning how to write electron configurations becomes easier once you understand fluorine’s example. The general rule is to fill orbitals in the following order:
-
1s
-
2s
-
2p
-
3s
-
3p
-
4s
-
3d
-
4p, and so on.
Following the Aufbau principle, electrons fill lower-energy orbitals first before moving on to higher ones. Fluorine’s simple structure makes it an excellent starting point for understanding this process.
Fluorine’s Electron Configuration and Its Impact on Bonding
1. Ionic Bonding
Fluorine often forms ionic bonds with metals such as sodium. In sodium fluoride (NaF), sodium donates one electron to fluorine, completing fluorine’s octet.
2. Covalent Bonding
In compounds with nonmetals like hydrogen, fluorine shares electrons. In hydrogen fluoride (HF), each atom contributes one electron to a shared pair, resulting in a strong covalent bond.
The electron configuration of fluorine is 1s² 2s² 2p⁵, showing that fluorine has nine electrons arranged in two shells. With 7 valence electrons, fluorine is only one electron short of having a full octet, explaining its strong reactivity and electronegativity.
Fluorine’s electron configuration not only defines its chemical behavior but also explains why it plays such a significant role in various industries and everyday life. From water fluoridation and toothpaste to non-stick cookware and refrigerants, fluorine’s ability to gain one electron makes it incredibly useful.
By understanding fluorine’s electron configuration, we gain insight into the very foundation of chemistry: how atoms interact, bond, and create the substances that shape our world.